Core Biotechnology Services
Services and techniques
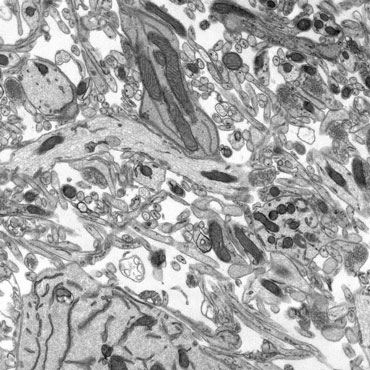 TEM - resin embedding and ultramicrotomy
TEM - resin embedding and ultramicrotomy
Resin embedding enables internal visualisation of larger samples such as tissue, cells and biopsies. This reveals how tissues or cells are organised, and differences in cell or organelle morphology.
The sample must be carefully processed through a bespoke procedure depending on the results desired. The process usually involves a series of steps:
- Stabilisation of the sample through chemical or cryogenic fixation
- Chemical treatment to introduce contrast to the sample when it is viewed under an electron beam
- Serial dehydration to replace all water with a transition solvent
- Infiltration with desired plastic resin
- Polymerisation to harden the cells embedded in the resin
- Ultramicrotomy to cut 70nm thick slices through the sample
The thin sections are collected onto 3mm support grids and viewed in the TEM. An electron beam is passed through the sample, projecting a 2D image of the tissue slice on a viewing screen.
Many modifications to the basic protocol can be applied to achieve many different goals, immunogold labelling for example; the in situ localisation of specific tissue constituents using antibody and colloidal gold marker systems. Cryo-ultramicrotomy is the ultrathin sectioning of fixed, cryo-protected, frozen hydrated samples at very low temperatures, a technique used when less harsh tissue processing is required - particularly useful in immunogold investigations.
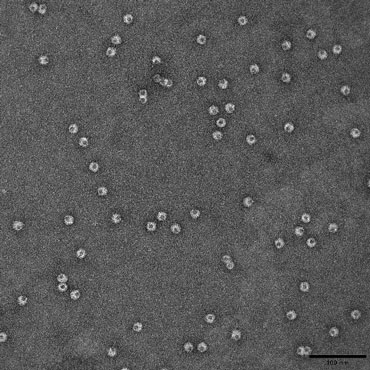 TEM - negative staining
TEM - negative staining
Negative staining is used to investigate the shape and size of isolated macromolecules, bacteria and virus.
Samples in solution are placed directly on a support grid that has a translucent support substrate stretched across it. Once the sample has adsorbed to the support, an electron dense contrasting agent is applied. The contrast agent is quickly blotted away, leaving a stain that is denser on the background support than the sample. This results in a ‘negative’ image of the sample.
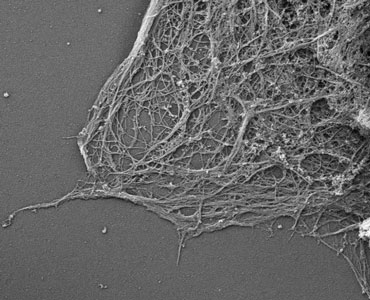 SEM
SEM
In this laboratory, we use the scanning electron microscope for high resolution surface topographical study of mainly, though not exclusively, biological specimens.
Bulk samples much larger than those used for transmission electron microscopy may be routinely prepared and imaged. The large depth of field achievable can produce an image of great visual depth with a three-dimensional appearance.
Typically, a specimen is chemically fixed, any water is removed by passing through a series of transition solvent, and then the sample is dried using equipment or chemistry that minimises distortion from dehydration aretfact. For dry samples, this process is not necessary. The samples are mounted/orientated on a stub of metal, coated with 40 - 60 nm of metal, and then observed in the SEM.