Institute for Precision Health
Past events
Innovation Clinics
Grow your innovations
Date: 10 June 2025
Leicestershire Innovation Clinics, are a unique opportunity to access expert support for innovation in the life sciences, medtech and health tech sectors. The event was hosted in partnership with Medilink Midlands, The Institute for Precision Health and the Charnwood Campus Science Innovation and Technology Park, leveraging expertise from Health Innovation East Midlands and the Leicestershire Academic Health Partnership. It was designed for individuals and companies in, or transitioning into, the life sciences sector within Leicester and Leicestershire.
The aim was to offer a clear, structured pathway for growing and developing new innovations, providing confidential discussions on the development of products, including:
- Advice on funding and product development
- Engagement with the NHS
- Clinical insights and regulatory advice
- Networking opportunities within the sector
If you would like to attend one of our upcoming sessions, please go to our events page
Struggling with UTIs?
Learn more about Leicester’s phage research
Date: 23 May
This talk was to help give an understanding about how we might be able to use Phages in antibiotic resistant urinary tract infections, led by the Centre for Phage Research's Dr Melissa Haines. Attendees were also able to give their thoughts on the use of Phages and what they perceive some of the barriers to be.
Watch the behind the scenes video (links to LinkedIn)
ELRIG UK Organoid Conference
Date: 8 May 2025
We were delighted to host the ELRIG (European Laboratory Research and Innovation Group) UK Organoid Conference. The meeting was opened by our Royal Society Entrepreneur in Residence, Dr Tim Hammonds, with the University's Dr Gareth Miles from the Explant Facility presenting ground-breaking approaches to 3D patient-derived models for cancer research.
Drug discovery is rapidly moving beyond the traditional immortalised cell lines into advanced models that can better mimic and predict the complex physiological environment of the human body. Organoids and spheroids have the potential to revolutionise drug discovery and toxicology by offering more predictive, human-relevant insights. This forum brought together experts from academia, biotech, and pharma to explore the latest advancements in physiologically relevant cell models, and how they are being adopted in practical applications.
The ELRIG organoid conference allowed us to reflect on the challenges and opportunities of integrating these advanced models into the drug development pipeline, with excellent presentations from both researchers and industry showcasing their state of the art solutions. Organoids and 3D models offer opportunities to improve drug candidate selection, reduce late-stage failures, and enhance the predictive power of preclinical testing.
Leicester Innovation Festival at Charnwood Campus
Accelerate innovation in Life Sciences - support and collaboration opportunities
Date: 3 April 2025
This was the seventh Leicester Innovation Festival event, in partnership with Charnwood Campus, Medilink Midlands and The Institute for Precision Health, designed to help life sciences businesses thrive.
Attendees were able to discover how businesses have successfully advanced new products with support from Leicestershire Innovation Clinics. Gain insights into available funding, regulatory, commercialisation expertise, and collaboration opportunities with the NHS and academic institutions in Leicestershire. Participate in expert drop-in sessions to help bring new product to market. Attendees were also able to expand their network with fellow innovators, clinicians, academics, and industry leaders.
Leveraging expertise in the College of Business - CoB/CLS joint event
Date: 2 April 2025
Research excellence in the College of Business has the facility to add business acumen, innovation pipelines, organisational and managerial dimensions to both targeted and interdisciplinary research grants across the life sciences and for wider healthcare delivery. This event will showcased key research themes driving impact across both colleges, with an ambition for enhanced collaboration and funding applications.
Joint BRC/IPH PPIE event to understand patient and family perspectives on living with Tuberculosis (TB)
Date: 22 March 2025
Dr Pranabashis Haldar and Professor Manish Pareek and their research teams alongside the BRC PPI team and IPH, are delivered an event that to help to educate about TB and Leicester's TB research, alongside exploring and developing ideas for improving engagement in PPI activities to help understanding around experiences of living with TB.
IPH's first outing with the Heritage Hub
Date: 15 February 2025
Location: Beaumont Shopping Centre, Beaumont Leys
IPH and the Heritage Hub supported the “Cabinet of Curiosity” event at the Beaumont Centre on the 15th of February. The Leicester Museums and Galleries, the Heritage Hub and IPH brought fascinating objects from technology and natural history collections which were examined up close. Creating cells in petri dishes was a firm favourite for the younger visitors, however the Heritage Hub's fantastic collection of archaeological finds and mini-beasts, combined with electron microscopy (EM) images of disease-causing micro-organisms created quite a crowd too.
This interactive exhibit had something to intrigue all ages.
Women's Health Research Forum
Date: 29 January 2025
The women's health research forum held at Leicester Tigers brought together for the first time, a wide variety of researchers specialising or interested in women's health and wellbeing research. We would like to thank all of the inspirational speakers and attendees for their engagement in helping us to define key research questions and to understand the research gaps. Once we map emerging areas of interest, we will share next steps and invite further input. Our next event will be specifically aimed at bringing early career researchers and PGR students into the conversation as we look to expand the network.
IPH PGR research pitching
Date: 1 November 2024
It was great to catch up with our IPH PGR students who gave short presentations on their fabulous projects. This gave opportunity to find out about the progress of the projects and future aims, in addition to understanding what events our students would like the Institute to deliver for them.
You can find out the titles of our PGR's projects on our research opportunities page.
Charnwood Campus presents Sustainability in Healthcare as part of the Leicester Business Festival
Date: 5 November 2024
Venue: Charnwood Campus Science and Technology Park
This was part of the many fabulous events that have taken place across Leicestershire as part of the Leicester Business Festival.
Health innovations and treatments that consider climate change, sustainability, and nature should be integral to functioning of a health system. Placing sustainability at the core of the future of healthcare offers opportunities to deliver better treatments, services, support healthier populations, and save costs. The NHS has committed to reaching net zero by 2040 for the emissions they control directly, and by 2045 for the emissions they influence, through the goods and services they buy from partners and suppliers. With all new NHS procurement in requiring a Carbon Reduction Plan or Net Zero Commitment it is essential the business selling to the NHS are actively working on reducing their carbon footprint.
Merck Curiosity Cube
Dates: 18-20 September
Read about Merck Curiosity Cube
Huge thanks to all of our volunteers who helped to deliver STEM outreach to inspire the next generation of scientists at Beaumont Leys School, the Madani Schools Federation, and Loughborough deLisle. We had a fun-packed 3 days, and were blown away by the level of engagement from the amazing students across all 3 schools.
We also had the pleasure of the Deputy Lord Lieutenant of Leicester, Ms Penny Coates joining us at Beaumont Leys. A huge thanks to the Merck Group and Charnwood Campus for giving us the opportunity to engage with our local schools.
MedTech@Leicester
Date: 4 September 2024
We were delighted to support the Biomedical Engineering Research Group (BERG) in bringing together MedTech expertise across Leicester partners and beyond.
This was a free event showcasing translation of clinical research at Leicester into innovative technologies with the potential to improve people’s lives and wellbeing.
Developing partnerships for innovation in health
Date: 27 June 2024
This was a free event which will brought together academics, clinicians, businesses and funders to showcase how the University of Leicester and Leicester Academic Health Partners enable health innovation using proof-of-concept funding throughout the pipeline of basic research through to clinical implementation.
This activity was enabled by our UK Research and Innovation (UKRI)-funded Impact Accelerator Accounts (IAAs) and in partnership with Medilink Midlands.
Joint IPH/LISCB event - Harnessing structural and chemical biology for translational research
Date: 18 April 2024
Structural and chemical biology plays an essential role in the translational research pipeline, enabling greater understanding of disease mechanisms and drug interactions that can facilitate disease prevention and treatment. Led by the Institute for Structural and Chemical Biology's director Professor John Schwabe, clinical structural biologist Professor Bibek Gooptu, and IPH's clinical director Professor Chris Brightling, this joint institute event showcased how structural and chemical biology contributes to health research.
We look forward to working together to strengthen the IPH/LISCB collaboration, developing research themes and applying for interdisciplinary funding.
IPH PGR talks day
Date: 15 April 2024
It was great to catch up with our IPH PGR students who gave short presentations on their fantastic projects. This gave opportunity to find out about the progress of the projects and future aims, in addition to understanding what events our students would like the Institute to deliver for them. We are already looking forward to our next IPH PGR seminar day in a few months' time.
You can find out the titles of our PGR's projects on our research opportunities page.
Joint IPH/BRC PPIE - Event for patients with multiple long term conditions
Date: 23 March 2024
Venue: GDC LT2 (capacity 200) + breakout rooms for each project
This event will be led by the Biomedical Research Centre (BRC) patient and public engagement team who will be refreshing their patient and public group membership and talking about upcoming projects across the BRC and the Institute. This will be a fantastic opportunity to engage our local community with the breadth of research being undertaken within the BRC and IPH.
Read more about the studies associated with this event
LLR ICB Health Data Science and Research Conference
Date: 12 March 2024
Venue: College Court Conference Centre
The objective of this day was to create a network to share knowledge and support each other to improve healthcare for our population by bringing analysts, clinicians and managers in the NHS together with academic colleagues who have expertise in data science and advanced analytic methods.
Innovation, productivity and health inequalities: Developing a road map for Leicestershire
Date: 6 February 2024
Venue: Charnwood campus
Innovation, productivity and health inequalities was the first life science dedicated day of the Leicestershire Innovation Festival.
This event gave opportunity to learn about the strategies for reducing health inequalities in Leicestershire and how innovation is helping to reduce this gap. Speakers included innovators, NHS, clinical leads and business who shared some world leading case studies.
Pitching your research
Date: 24 November 2023 Venue: College Court
The Institute for Precision Health held a workshop for PGRs and ECRs to give a better understanding of how to pitch your research successfully and succinctly, directly feeding into IAA funding pitches. This was the first in a series of workshops to help improve research communication and build confidence.
This was open to all PGRs and ECRs from across all departments.
Our speakers on the day were Dr Lynne Howells - Introduction, Professor Don Jones - Preparing your pitch, Dr Carl Edwards - Understanding intellectual property and commercialisation steps and Dr Rebecca Hames - Writing for impact and understanding funding requirements.
Some of our attendees gave excellent 2 minute pitches for their project ideas. Our winner on the day was Dr Colleen Maxwell (CVS).
Research Institutes and Centres – strategies and opportunities
Date: 26 October 2023
Venue: Digital Culture Studio, David Wilson Library
The purpose of this event was to foster collaboration and alignment among the University's Research Centres and Research Institutes and explore interdisciplinary opportunities within and beyond our colleges. The event also gave opportunity to learn about the strategies and support available from the Institutes and learn about the current and future funding landscape from the Research, Partnership and Development (RPD) team.
The agenda consisted of:
- Institute director and representative talks about strategy and support available
- The RPD team gave an overview of funding opportunities with emphasis on interdisciplinarity/opportunities from key funder working groups
- A facilitated discussion about the alignment of all centres across the institutes, the ideas that map across institutes and the ideas that align with upcoming calls
Are You Industry Ready? Transitioning from academia to industry and growing your career
Date: 7 September 2023
Venue: Charnwood Campus – Loughborough
A masterclass for early career life science research scientists looking to move from academia into industry. The day consisted of facilitated interactive workshops run by professional coaches Engage and Grow and Action Coach in conjunction with industry speakers, led by Quotient Sciences.
This full day event was aimed at PGR students and ECR’s within the life sciences, chemistry, computing and mathematical sciences from across the midlands to explore what industry recruiters are looking for, understand the value of their transferable skills and build confidence to explore new career opportunities.
Workshops and presentations included how to:
- Leverage your transferable skills to boost employability
- Ace job interviews: Gain insights for interview skills and leave with confidence
- Develop your career pathway: Be clear on next steps for career success
- Network for success: Connect with industry experts, build relationships and explore opportunities
Follow us on X and LinkedIn to see posts from the day.
Collaboration in Data Science and Radiotherapy 1st and 2nd workshop
NIHR Leicester BRC, University of Leicester and Institute for Precision Health
Dates: 12 May 2023 and 23 June 2023Following the first introductory workshop, the aim of the 2nd all-day workshop was to bring together combined expertise in radiotherapy, data science, AI and machine learning and to develop collaborations and work on project ideas with a view to future funding applications. Datasets available for analysis including local data were also discussed.
The first half-day workshop took place on 12 May 2023 and identified areas or problems of interest (see the list of potential projects below). These were presented again at the second all-day workshop at College Court.
List of potential projects:
- Identifying patients at risk of radiotherapy-associated pneumonitis in lung cancer using a radiomics approach
- Impact of image quality parameters and data harmonization on machine learning accuracy
- Predicting patient outcomes from radiotherapy planning CT imaging features
- Impact of brainstem- and hippocampus-sparing radiotherapy on function in brain radiotherapy
- MRI predictors of local recurrence and distant relapse following neoadjuvant chemotherapy for breast cancer
- Clinical and imaging features predicting feeding tube use in radiotherapy for head & neck cancers
- CT image classification and compression for data analysis
- Applying multiple-model and ensemble learning methods to multi-output or multi-class problems in radiotherapy
QIAGEN's 2023 Cancer Research roadshow
Date: 27 April 2023
A Roadshow Exhibition featuring new Instrumentation solutions for Cancer Research
QIAGEN Specialists were available for Q&A and advice sessions and Lunchtime talks were held by our specialists and Research Customers.
- Introduction and QIAGEN Cancer Solution Overview – Cara Hall, Senior Customer Solution Manager
- Liquid Biopsy Biomarkers – Rebecca Allsopp, University of Leicester
- Enhancing Sensitivity and Precision in Mutation Detection with QIAcuity Digital PCR – Imran Kibria, Senior Instrument Specialist
- Cutting edge sequencing for challenging RNA samples – Syed Abedi, NGS Specialist
Driving Innovation in Midlands Healthcare
Date: 25 April 2023
A Charnwood Campus Life Sciences Cluster event in partnership with the University of Leicester.
This event took place on the Charnwood Campus and aimed to facilitate development of research partnerships between Industry and the University of Leicester.
Our speakers were:
- Professor Chris Brightling, Co-Director University of Leicester Institute for Precision Health, Honorary Consultant Respiratory Physician, Respiratory Theme Lead for Leicester NIHR Biomedical Research Centre (Respiratory).
- Professor Jacqui Shaw, Co-Director University of Leicester Institute for Precision Health, Head of Department Genetics and Genome Biology (Genetics).
- Professor Pratik Choudhary, Honorary consultant in Diabetes, Leicester Diabetes Centre. Chair of Diabetes Technology Network UK.
- Dr James Hodgkinson, Associate Professor of Chemistry and Chemical Biology, School of Chemistry.
- Professor Don Jones, Co-Director University of Leicester Institute for Precision Health, Director of van Geest MultiOMICS facility and Dr Jim Langridge, Waters (Biomarkers).
- Catalent - Industry Showcase Talk.
- Professor Andre Ng, Head of Department, Cardiovascular Sciences, Professor of Cardiac Electrophysiology, Consultant Cardiologist and Electrophysiologist.
- Dr Lucy Alexander, Head of Business Development, Charnwood Campus.
- Dr Tim Hammonds, Royal Society Entrepreneur in residence, Honorary Visiting Fellow
Download the agenda (docx, 712kb).
See our Pro Vice-Chancellor for Research and Enterprise Professor Phil Baker talking about research at Leicester in our launch video.
Institute for Precision Health PGR Seminar Day
Date: 10 March 2023
This event was open to institute-funded PhD students and their supervisors.
This event allowed IPH funded students to share their research projects and their contributions to IPH with the other Institute students, supervisors and Institute directors. Each gave a 10 minute presentation and answered questions outlining their research.
There was also an opportunity to win a prize for the best image submitted.
Congratulations to Gemma Donaldson (awaiting approval to publish the image) and Savvas Papageorgiou, our joint winners who received £25 each in vouchers.
You can see the image submitted by Savvas below (Image by the Advanced Imaging Facility (RRID:SCR_020967) at the University of Leicester and the BBSRC grant nr: BB/S019510/1). The image shows the identification of point mutations within EML4-ALK V3 that cause dissociation of the oncoprotein from microtubules. Beas2B parental cells were transfected with YFP-tagged EML4-ALK V3 constructs and stained with antibodies against GFP (in green) and α-tubulin (in magenta). Phosphomimetic constructs (S144D/S146D and S134D/S144D/S146D V3) exhibit less localization to microtubules compared to wild-type V3 (P = <0.0001) and similar microtubule localization with cells lacking EML4-ALK V3 entirely (i.e., YFP-only) (P = 0.9983 and P=0.9997, respectively). Images were taken using the Airyscan high resolution microscope at 63X magnification.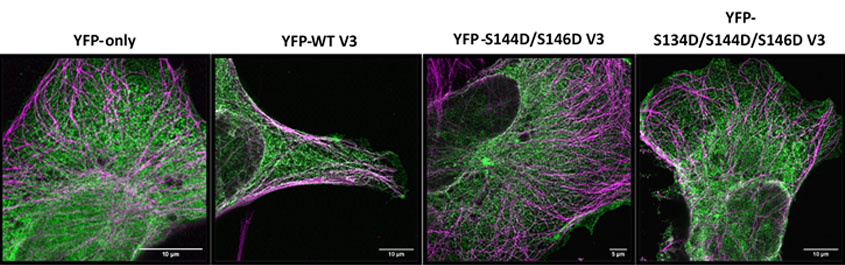
Improving health outcomes through data science, AI, machine learning, mathematical modelling and biomedical engineering
Professors Chris Brightling, Jacqui Shaw, Don Jones (Institute for Precision Health) and Professor Simon Gill (Chair in Theoretical Mechanics, College Dean for College of Science and Engineering) will bring together an interdisciplinary team to understand how our data scientists, computer scientists, mathematical modellers and engineers can contribute to innovative solutions in areas of unmet clinical need and wider healthcare challenges.
The first steps were to map out the areas of expertise and interest across the University of Leicester, Leicester NIHR-Biomedical Research Centre, University Hospitals of Leicester and University Hospitals of Northamptonshire.
Applications were made (closed 28 February 2023) so that our first seminar and networking event around areas of common interest could be arranged.
For more information email Dr Lynne Howells (iph@le.ac.uk).
Improving the research culture around Black and/or South Asian communities: improving accessibility to cancer clinical trials (external event)
This event provided an opportunity to explore the challenges to participation in research/cancer clinical trials in Black and South Asian communities. As part of this event, a series of group discussions was organised involving experts by experience, charity representatives and researchers to co-produce a strategy that identifies priorities to improve inclusion in research to tackle health inequality. From identifying barriers to inclusion and how we achieve a diverse and inclusive research culture, to improving how we understand patients’ needs and expectations and how we can reach out to Black and/or South Asian groups in our geography.
Speakers
- Shahnaz Aziz, Patient Public Leadership and Equality Lead, East Midlands Academic Health Science Network
- Dr Oladejo Olaleye, University Hospitals of Leicester NHS Trust
Date: 24 February 2023
Equality, Diversity and Inclusion in Cancer Care and Research
This event was open to internal staff, organised by Gianina-Ioana Postavaru and took place on 27 January 2023
The first part of this event involved a short (10-15 min) presentation from UoL/UHL colleagues to showcase their research in the area. They presented research areas/questions they would like to develop and invite collaborators. The second part provided an opportunity for discussions to develop research ideas around EDI in cancer care and form new collaborative links.