School of Chemistry
PhD opportunities
The School of Chemistry is proud to host postgraduate research students from diverse backgrounds, and offers a broad range of possibilities for PhD study to both UK and international students. In addition to regularly-updated funded opportunities, we welcome applications at any time from qualified students that are able to self-fund their studies, for example through scholarships in their home countries.
A selection of recently advertised PhD projects are listed below, covering all of the research themes within the School of Chemistry:
- Chemical Biology
- Leicester Chemical Learning Enhancement and Pedagogy
- Materials and Interfaces
- Spectroscopy and Atmospheric Chemistry
- Sustainable Synthesis and Catalysis
Please see how to apply, and feel free to contact our academic staff to discuss these opportunities.
Enantioselective Fluorinations with the Hypervalent Fluoroiodane Reagent
Project supervisor
Project details
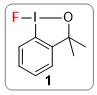
An important strategy in the drug discovery process is the incorporation of fluorine into biologically-active molecules because fluorine can increase the potency and improve the pharmacokinetic properties. Consequently, 30% of all agrochemicals and 25% of all pharmaceuticals contain fluorine atoms. In 2013 we introduced the hypervalent iodine(III) reagent 1 as a new, easy-to-handle fluorinating reagent for installing carbon-fluorine bonds. Initially, a transition metal was required to activate the fluoroiodane reagent 1 by coordinat-ing to the fluorine atom, but in 2019 we demonstrated that it can be activated by hydrogen bonding to hexafluoroisopropanol. The aim of this exciting new research project is to combine chiral hydrogen bond donors with the fluoroiodane reagent 1 to develop enantioselective fluorinations.
The successful candidate will gain hands-on-experience in synthetic organic chemistry, asymmetric catalysis, reaction design, molecular modelling and modern analytical techniques using state-of-the-art equipment (multinuclear NMR spectroscopy, stopped-flow NMR spectroscopy, mass spectrometry, chiral GC and chiral HPLC). This PhD project will provide excellent training for a student interested in a career in either academic or industrial research such as in synthetic methodology development, medicinal chemistry, agrochemistry, process chemistry, as well as in fine and speciality chemicals.
References
- “Alkene vicinal difluorination: From fluorine gas to more favoured conditions” S. Doobary and A. J. J. Lennox, Synlett, 2020, 31, 1333-1342.
- “Electrophilic fluorination using a hypervalent iodine reagent derived from fluoride” G. C. Geary, E. G. Hope, K. Singh and A. M. Stuart*, Chem. Commun., 2013, 49, 9263-9265.
- “Intramolecular fluorocyclizations of unsaturated carboxylic acids with a stable hypervalent fluoroiodane reagent” G. C. Geary, E. G. Hope and A. M. Stuart*, Angew. Chem. Int. Ed. Engl., 2015, 54, 14911-14914.
- “ Activation of the hypervalent fluoroiodane reagent by hydrogen bonding to hexafluoroisopropanol ” H. K. Minhas, W. Riley, A. M. Stuart* and M. Urbonaite, Org. Biomol. Chem., 2018, 16, 7170-7173.
- “Accessing novel fluorinated heterocycles with the hypervalent fluoroiodane reagent by solution and mechanochemical synthesis” W. Riley, A. C. Jones, K. Singh, D. L. Browne and A. M. Stuart*, Chem. Commun., 2021, 10.10139/d1cc02587b.
Further information
To apply: Applications for this position are now closed, check back soon for our latest PhD vacancies.
Informal enquiries to Dr Stuart are welcome. For further application details, please contact postgraduate admissions (chempgr@le.ac.uk).
‘Payload-Releasing Electrophiles’ – a new disease-selective delivery strategy for diagnostics and therapeutics
Project supervisor
Project details
Diseased cells such as cancer cells often contain many surface-exposed cysteine residues on proteins due to mutations, over-expression and/or protein mis-folding. Targeting these nucleophilic cysteinyl thiols with electrophiles is well-established and has been used to alter the functions of cysteine-containing proteins and to treat disease. We intend to build on this approach by developing electrophilic chemicals that can additionally release a ‘payload’ after their reaction with cysteines (payload-releasing electrophiles, PREs). These PREs will enable the specific delivery of imaging agents or drugs to diseased cells.
This new PRE cell targeting concept would not only induce therapeutically relevant modifications on the target proteins, but would also allow the efficiency of modification to be monitored in real time (e.g. by releasing fluorophores). This would be very useful for diagnostic applications and for drug validation studies. If the payload were a drug molecule, the method would also enable co-operative treatments that would (i) improve overall efficacy, (ii) would avoid the emergence of drug resistance (a common issue with cysteine-modifying drugs), and (iii) would reduce toxicity to healthy cells. This latter point is particularly important for cancer therapy, as the off-target toxicity of many anti-cancer agents precludes their clinical use. By incorporating electron-withdrawing groups on the PRE, we should also be able to induce reversible modification of the target protein, which in turn would enable catalytic generation of the payload. This would overcome any dosing issues associated with protein-induced payload release. We therefore propose that PREs have unprecedented potential to improve therapies for cancer and other diseases.
The project will involve chemical synthesis (including structure design), recombinant protein expression and purification, NMR- MS- and fluorescence-based enzyme inhibition assays, and human tissue culture. Therefore, this highly multidisciplinary project will give an excellent grounding in biochemical and biomedically relevant techniques, and will ultimately produce a highly skilled multidisciplinary scientist. Training will be provided for all experimental methods.
Further information
To apply: Applications for this position are now closed, check back soon for our latest PhD vacancies.
Informal enquiries to Dr Hopkinson are welcome.
Mechanochemical Synthesis for Sustainable Chemical Manufacturing
Project supervisor
Project details
There is an urgent need to break our reliance on precious metals (e.g. Pd, Pt) in the chemical industry owing to their scarce availability, high costs and sustainability concerns. AE alkaline earth metals are very environmentally benign and cheap (Mg £2/kg, Ca £2/kg, Ba £20-50/kg); crucially, low-valent AE metals possess physicochemical properties suitable to replace precious metals, modernise and improve efficiency in the chemical industry, as they have already shown great potential in the activation of small molecules of great relevance for chemical engineering applications (e.g. N2, Haber-Bosch; CO/H2, Fischer-Tropsch). Despite their great promise, their applications have been blocked by the challenging preparation of low-valent AEs.
This project aims to address key issues which threaten the long-term sustainability of chemical engineering and manufacturing. The PhD candidate will deliver the first facile synthesis of low-valent AE electrides (i.e. materials where electrons are delocalised and not associated with well-defined sites/atoms) and unlock their application as earth-abundant alternatives to precious metals that will transform the chemical industry. The work will be based on the innovative use of mechanical forces (i.e. mechanochemistry) developed by the Ortu Group, in which we have shown that simple AE amide precursors (e.g. [Ca{N(Mes)(SiMe3)}3K] can be converted into highly reactive AE electrides (I, Figure 1) via quick solvent-free synthesis with minimal manipulations; these species are able to perform facile C-H activation chemistry and pyridine coupling (work currently under consideration with Nature Communications). Crucially, our mechanochemical methods can significantly reduce the use of hydrocarbon solvents and deliver quick and scalable multi-gram synthesis under very mild conditions. The PhD candidate will be to build on these exciting preliminary results by developing new AE electrides and targeting strategic reactions for the chemical industry and fine chemical production (e.g. Fischer-Tropsch, Haber-Bosch, C-C coupling).
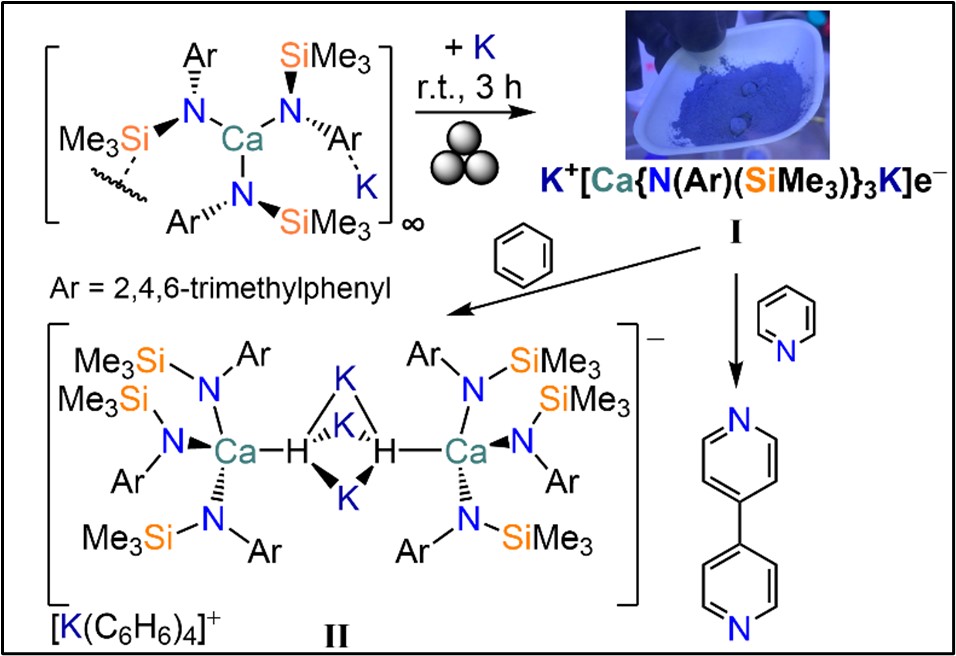
Figure 1. Synthesis of a Ca electride (I) and its reactivity.
AE electrides will be synthesised using mechanochemical methods, supported by state-of-the-art anaerobic techniques (Schlenk line and glovebox), advanced organometallic and organic synthesis. The student will be involved in the physical characterisation of the new materials (EPR, electron-conductivity, XRD, XAS, RINXS) working alongside national and international collaborators.
Further information
To apply: Applications for this position are now closed, check back soon for our latest PhD vacancies.
Informal enquiries to Dr Ortu are welcome. For further application details, please contact postgraduate admissions (chempgr@le.ac.uk).
Chemical energy conversion in biology studied using advanced spectroscopic and structural tools
Project supervisor
Project details
Redox properties of metal-containing active sites are critically important to many biocatalytic processes: one third of all proteins contain a redox-active metal, and ca 22% of submissions to the Protein Data Bank contain a transition metal. Metalloproteins capable of extracting energy from H2 gas, sequestering CO2 from the atmosphere, or performing complex monooxygenation reactions, rely upon the ability to access and control a range of often exotic metal oxidation states in an aqueous environment. Much of this crucial chemistry occurs at extremely fast rates, making it challenging to study using conventional structural and spectroscopic methods.
This project aims to investigate the catalytic mechanisms and structural dynamics of metalloenzymes that are vital for chemical energy conversion, with a focus on hydrogenase. State-of-the-art spectroscopic and structural studies will be combined with computational analysis to reveal critical but elusive transient intermediates by studying reactions in real time on sub-microsecond timescales. The outcomes of this project will provide a step change in our understanding of the mechanism of hydrogenase and other metalloenzymes, and will serve as inspirational catalysts for future green energy technologies.
The PhD student will gain a broad range of interdisciplinary skills in spectroscopy, electrochemistry, chemical biology, structural biology, and biophysics whilst addressing critical questions about how nature achieves efficient chemical energy conversion.
Further information
To apply: Applications for this position are now closed, check back soon for our latest PhD vacancies.
Informal enquiries to Dr Ash are welcome.