Sustainable synthesis and catalysis
Organofluorine chemistry
 Fluorine chemistry has influenced progress significantly in science and technologies developed over the past century and has commercial applications in pharmaceuticals, agrochemicals and material science. Fluorine is the most reactive element in the periodic table, however some organofluorine compounds have chemical inertness likened to that of the noble gases. Therefore, the field of organofluorine chemistry is full of quirks, defined by contrasting chemistries and overall is a fascinating field to study.
Fluorine chemistry has influenced progress significantly in science and technologies developed over the past century and has commercial applications in pharmaceuticals, agrochemicals and material science. Fluorine is the most reactive element in the periodic table, however some organofluorine compounds have chemical inertness likened to that of the noble gases. Therefore, the field of organofluorine chemistry is full of quirks, defined by contrasting chemistries and overall is a fascinating field to study.
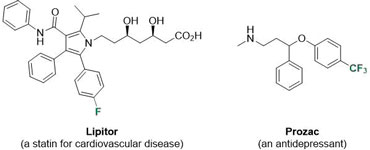 The incorporation of fluorine has proved crucial in the development of new pharmaceutical products because a single fluorine atom can alter the electronics of a molecule without major steric implications. Fluorine is often introduced into drug candidate molecules to increase the metabolic stability, modulate the compound’s conformation and alter the lipophilicity leading to enhanced bioavailability. Hence, approximately 30% of leading blockbuster drugs, such as Lipitor and Prozac, contain fluorine and the chemical industry still needs more efficient methods for fluorination.
The incorporation of fluorine has proved crucial in the development of new pharmaceutical products because a single fluorine atom can alter the electronics of a molecule without major steric implications. Fluorine is often introduced into drug candidate molecules to increase the metabolic stability, modulate the compound’s conformation and alter the lipophilicity leading to enhanced bioavailability. Hence, approximately 30% of leading blockbuster drugs, such as Lipitor and Prozac, contain fluorine and the chemical industry still needs more efficient methods for fluorination.
 At Leicester, we are particularly interested in designing new synthetic methodology for the introduction of fluorine into organic molecules. Current research topics are:
At Leicester, we are particularly interested in designing new synthetic methodology for the introduction of fluorine into organic molecules. Current research topics are:
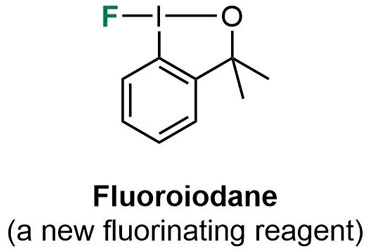
- Fluorinated heterocycles - Fluoroiodane is a new fluorinating reagent which was developed at Leicester. We are currently extending the substrate scope of this hypervalent iodine(III) reagent and using it to produce novel fluorinated heterocycles which cannot be accessed from traditional fluorinating reagents such as Selectfluor.
- New fluorinating reagents – Using the basic structure of fluoroiodane, we are preparing a new family of fluorinating reagents by changing the sidearms and substituents on the aromatic ring.
- Enantioselective fluorinations – We are also investigating new approaches to hypervalent iodine chemistry to prepare enantiopure fluorinated heterocycles and drug-type targets.
Selected Publications
- "Fluorinations of unsymmetrical diaryliodonium salts containing ortho-sidearms; influence of
sidearm on selectivity", A. M. H. Abudken, E. G. Hope, K. Singh and A. M. Stuart, Org. Biomol. Chem., 2020, 18, 6140-6146. DOI:10.1039/D0OB01401J. - "Activation of the hypervalent fluoroiodane reagent by hydrogen bonding to hexafluoroisopropanol", H. K. Minhas, W. Riley, A. M. Stuart and M. Urbonaite, Org. Biomol. Chem., 2018, 16, 7170-7173. DOI:10.1039/C8OB02236D.
- "Intramolecular fluorocyclisations of unsaturated carboxylic acids with a stable hypervalent fluoroiodane reagent", G. C. Geary, E. G. Hope and A. M. Stuart, Angew. Chem. Int. Ed., 2015, 54, 14911-14914. DOI:10.1002/anie.201507790.