People
Dr Robert Mahen
Lecturer
School/Department: Molecular and Cell Biology, Department of
Email: rm722@leicester.ac.uk
Research
Life is sustained by the collective function of subcellular molecular machines termed organelles. Much is still to be understood about the assembly and function of organelles – a question which is critical to human health and disease.
We seek to understand the fundamental functions of centrosomes – microtubule-based organelles that allow cells to divide, move and sense their environment. Defects in centrosomes cause many poorly understood developmental diseases and are also implicated in carcinogenesis. Our work uses combined tools from fluorescence microscopy, genome editing and biochemistry, to understand the function of centrosomes across scales, with a long-term goal to treat human disease.
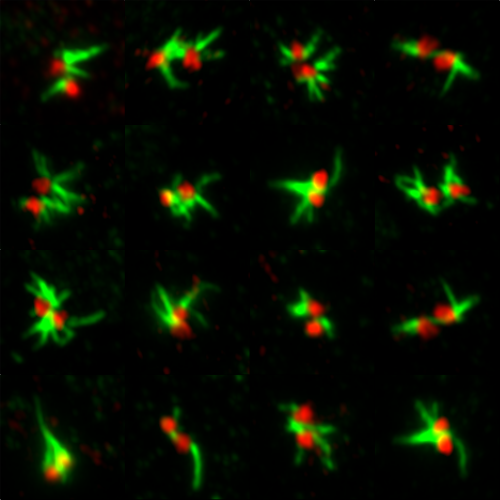
Fluorescence imaging of centriolar rootlets.
During cell division centrosomes form spindle poles, and thus are important for accurate chromosome segregation. My work has developed technologies to study centrosomes directly inside living cells, and proposed new models for how centrosomes are constructed from component parts during mitosis. Centrosomes also form cilia: hair-like appendages used for cell sensing and motion in a range of different human tissues. My recent work has pioneered imaging of cytoskeletal fibres nucleated by centrosomes termed rootlets, with a view to understanding human ciliary structure and function. We are collaborating with groups from across the University of Leicester to understand centrosome functions in human tissues from a multidisciplinary perspective.
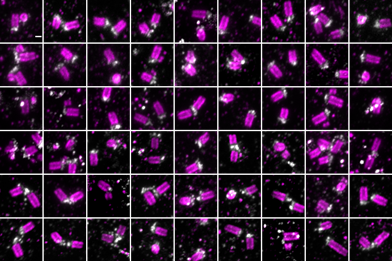
Expansion microscopy imaging of centrioles
Publications
Mahen, R. cNap1 bridges centriole contact sites to maintain centrosome cohesion. PLOS Biology. 2022. 20(10).
Mahen, R. The structure and function of centriolar rootlets. Journal of Cell Science. 2021. 134(16).
Lee M, Shorthouse D, Hall B, Mahen R, Venkitaraman AR. Cancer causing BRCA2 mutations disrupt an intracellular protein assembly mechanism to disable genome maintenance. Nucleic Acids Research. 2021. 49(10).
Nicolai S, Mahen R, Raschella G, Marini A, Pieraccioli M, Malewicz M, Venkitaraman AR, Melino G. ZNF281 is recruited on DNA breaks to facilitate DNA repair by non-homologous end joining. Oncogene. 2020. 39(4).
Sharma P, Mahen R, Rossman M, Stokes, JE, Hardwick B, Huggins D, Emery A, Hyvonen M, Spring DR, McKenzie GJ, Venkitaraman AR. Targeting a cryptic hydrophobic pocket in the polo-box domain of the polo-like kinase PLK1 to regulate mitosis. Nature Scientific Reports. 2019 9(1).
Mahen R. Stable centrosomal roots disentangle to allow interphase centriole independence. PLOS Biology. 2018. 16(4).
Mahen R, Koch B, Wachsmuth M, Politi AZ, Perez-Gonzalez A, Mergenthaler J, Cai Y, Ellenberg J. Comparative assessment of fluorescent transgene methods for quantitative imaging in human cells. Molecular Biology of the Cell. 2014 25(22).
Mahen R, Hattori H, Lee M, Sharma P, Jeyasekharan AD, Venkitaraman AR. A-type lamins maintain the positional stability of DNA damage repair foci in mammalian nuclei. PLOS one. 2013 8(5).
Mahen R, & Venkitaraman, AR. Pattern formation in centrosome assembly. Current Opinion in Cell Biology. 2012 24(1).
Mahen R, Jeyasekharan AD, Barry NP, Venkitaraman AR. Continuous polo-like kinase 1 activity regulates diffusion to maintain centrosome self-organization during mitosis. Proceedings of the National Academy of Sciences, USA. 2011 108(22).