Virtual Genetics Education Centre
Genetics and law for higher education
This section covers genetics-related issues that are in the news and where the legislation has affected how they are used.
Topics
- DNA fingerprinting
- The UK National DNA Database
- Human embryonic stem cell research
DNA Fingerprinting
DNA fingerprinting was invented here at the University of Leicester by Professor Sir Alec Jeffreys in 1984. It was first used as evidence in 1987 to convict baker Colin Pitchfork, who was the first criminal caught using DNA fingerprinting in Leicester. It was also used to clear the original suspect Richard Buckland.
Uses of DNA Fingerprinting
DNA fingerprints are useful in several applications of human health care research, as well as in the justice system.
- Biological evidence
- Wildlife forensics
- Establishment of paternity
- Person identification
- Diagnosis of inherited disorders
- Developing cures for inherited disorders
The United Kingdom National DNA Database (NDNAD)
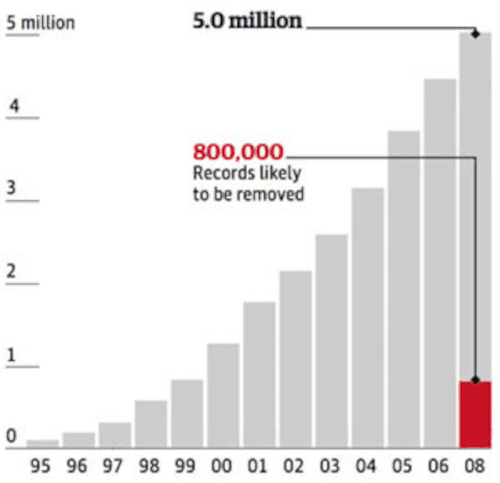
The UK NDAD a national DNA Database set up in 1995. At the end of 2005, it carried the profiles of around 3.1 million people, over 585,000 of them taken from children aged under 16. At the end of 2006, this figure had risen to more than four million records, making it the world's biggest DNA database at the time. The database, which grows by 30,000 samples each month, is populated by samples recovered from crime scenes and taken from police suspects and (in England and Wales) anyone arrested and detained at a police station, even if they are not subsequently charged with an offence.
The UK NDNAD is run by the Forensic Science Service (FSS), under contract to the Home Office.
Census data and Home Office statistics indicate that almost 40% of black men have their DNA profile on the database compared to 13% of Asian men and 9% of white men.
Controversy and the United Kingdom National DNA Database
The use of the database for genetic research without consent has been controversial, as has the storage of DNA samples and sensitive information by the commercial companies which analyze them for the police.
Given the privacy issues, but set against the usefulness of the database in identifying offenders, some have argued for a system whereby the encrypted data associated with a sample is held by a third, trusted, party and is only revealed if a crime scene sample is found to contain that DNA. Such an approach has been advocated by Prof. Sir Alec Jeffreys.
Others have argued that there should be time limits on how long DNA profiles can be retained on the Database, except for people convicted of serious violent or sexual offenses. GeneWatch UK has launched a campaign calling on people to reclaim their DNA if they have not been charged or convicted of a serious offense, and has called for more safeguards to prevent misuse of the database. The Human Genetics Commission has argued that individuals' DNA samples should be destroyed after the DNA profiles used for identification purposes have been obtained.
In May 2009, Britain bowed to a court ruling and promised to remove the DNA records of hundreds of thousands of innocent people from the NDNAD - but many will have to wait up to 12 years for their details to be deleted. People arrested on suspicion of minor offenses, such as shoplifting or public drunkenness, will have their DNA profiles held for six years even if they are not charged. The graph depicts the DNA of under 18's stored on the the UK NDNAD (source: HANSARD).
Human Embryonic Stem Cell Research
Stem cell research holds great promise for the development of new therapies for many serious human diseases. However, currently, clinically proven stem cell-based treatments have been established only for very few conditions, such as hematopoietic stem cell transplants for leukemia and epithelial stem cell-based treatments for burns and corneal disorders. All other stem cell applications are currently experimental.
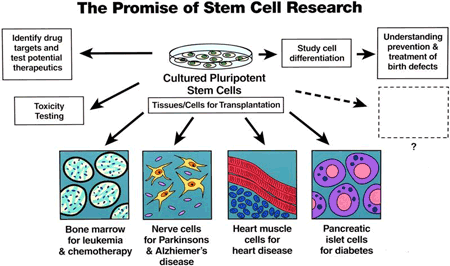
Stem Cell use in the UK
In the UK, the law states that the use of embryos in stem cell research can only be carried out with authority from the Human Fertilisation and Embryo Authority (HFEA). Licences are only granted if the HFEA is satisfied that any proposed use of embryos is absolutely necessary for the purposes of the research.
Research on human embryos is only allowed for certain purposes, which are outlined in the Human Fertilisation and Embryology Act (1990) and in the subsequent Human Fertilisation and Embryology (Research Purposes) Regulations 2001.
Human embryonic stem cell research is allowed only in the following conditions:
- To promote advances in the treatment of infertility
- To increase knowledge about the causes of congenital disease
- To increase knowledge about the causes of miscarriages
- To develop more effective techniques of contraception
- To develop methods for detecting the presence of gene or chromosome abnormalities
- To increase knowledge about the development of embryos
- To increase knowledge about serious disease
- To enable any such knowledge to be applied in developing treatments for serious disease
Licensed research can only take place on embryos created in vitro, i.e. embryos that have developed from eggs fertilised outside the body. Most embryos used in UK stem cell research are embryos initially created for use in fertility treatment, but which are not used. These "surplus" or "supernumerary" IVF embryos, if donated with full consent of the parents, can be used for research.
Licensed research can only take place on embryos up to 14 days. Stem cells are isolated from the blastocysti much sooner than this – at 5-6 days.
Human reproductive cloning is illegal in the UK. As a result of the Human Reproductive Cloning Act (2001) nobody in the UK is allowed to use cell nuclear replacement, or any other technique, to create a child.
Stem Cell use in Europe
The EU's 25 member states take different regulatory positions on human embryonic stem cell research, which reflects the diversity of ethical, philosophical and religious beliefs throughout Europe. These differences are reflected in the laws of each country,
Belgium has a similar legal position to the UK – allowing the procurement of human embryonic stem cells from surplus IVF embryos and, in particular circumstances (e.g. to study a particular serious disease), the creation of human embryos for the procurement of human embryonic stem cells.
At the other end of the spectrum, Germany and Italy prohibit the procurement of human embryonic stem cells from human embryos, while Austria, Bulgaria, Stem Cell use in EuropeCyprus, Ireland, Lithuania, Luxembourg, Malta, Poland, Romania and Slovakia have no specific legislation at all in this area.
Stem Cell use in the USA
In the US, legislation and funding for stem cell research are closely entwined. Until March 2009, at a federal level, scientists could not use government money to create new embryonic stem cell lines. All publicly funded work was confined to the 61 stem cell lines in existence in 2001, when the ban on deriving new lines was implemented.
In July 2006 President Bush vetoed a Bill lifting that ban, based on his opposition to the use of public funds for projects involving the destruction of human embryos. However, individual states have always had the authority to pass laws to permit human embryonic stem cell research using state funds. Several states have changed their legislation accordingly, including Connecticut, Massachusetts, California, and Illinois. This enabled the establishment of California's $3 billion Institute for Regenerative Medicine.
Private funding of embryonic stem cell research in the US has never been prohibited – leaving this sector largely unregulated.
However, with the arrival of President Barack Obama, the funding situation for stem cell research has changed dramatically, as he has lifted restrictions on federal funding for research on new stem cell lines. Mr Obama signed an executive order in a major reversal of US policy, pledging to "vigorously support" new research.