People
Dr Richard Doveston
Associate Professor of Chemical Biology, Postgraduate Research Admissions Tutor, and Chemistry MSc Coordinator

School/Department: Chemistry, School of
Telephone: +44 (0)116 229 7116
Email: r.g.doveston@leicester.ac.uk
Profile
I obtained a MChem degree in Pharmaceutical Chemistry here at University of Leicester in 2008 before going on to complete a PhD in the group of Professor Richard Taylor at the University of York. My PhD involved developing a synthetic route to the natural product janoxepin. Following this I took up a post-doctoral position with Professor Adam Nelson and Professor Steve Marsden at the University of Leeds to work in the area of ‘lead-oriented synthesis’. I then joined the Chemical Biology Group at the Technical University of Eindhoven (NL) and was there awarded a Marie Curie Fellowship in 2016. My research carried out under Professor Luc Brunsveld and Dr Christian Ottmann was focused on the discovery and evaluation of novel bioactive small molecules with a particular interest in using molecular glues to stabilise protein-protein interactions. I established my research group in the School of Chemistry and Leicester Institute of Structural and Chemical Biology in 2018. We are interested in continuing to understand and exploit the chemistry behind molecular glues in a pharmaceutical context. We take an interdisciplinary approach working closely with structural biologists and cell biologists.
Research
Research group - Stabilising protein-protein interactions
Protein-protein interactions (PPIs) represent an exciting yet challenging class of drug target to have emerged over recent years. With over 300,000 PPIs estimated in humans, and many shown to be implicated in disease, the discovery of small-molecule modulators of these interactions is of great interest. Whilst a vast number of de novo PPI inhibitors such as the Nutlins have been successfully developed, only a handful of complex natural product-derived PPI stabilisers such as paclitaxel (Taxol) have been exploited in the clinic (although to great effect). The number of reported synthetic stabilisers is growing, but the mode-of-action for the vast majority of these examples has been defined in a post hoc fashion. The development of rational strategies for the discovery of de novo PPI stabilisers therefore represents a significant and as yet unmet scientific challenge.
My research is focused on developing chemical biology approaches that will allow us to meet this challenge. Specifically, my interests lie in molecules that act at a given PPI interface and thus (might) act like a ‘molecular glue’. Underpinned by synthetic organic chemistry, two core research avenues focus on enhancing our molecular understanding of PPI stabilisation and establishing improved ligand-discovery techniques.
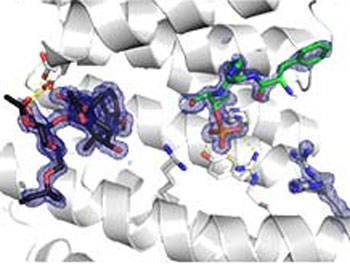
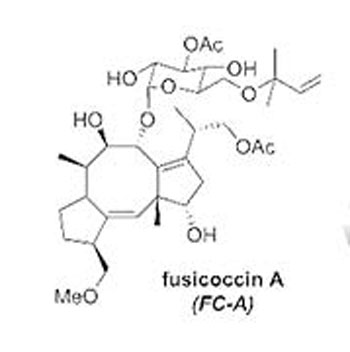
Better understanding of PPI stabilisation at the atomic level is essential in order to design more synthetically tractable, selective and potent small molecules. To achieve this a combination of in silico evaluation, synthesis, biophysical and structural evaluation is being used to enhance our understanding of natural product PPI stabilisers. As a case in point, the mechanism for fusicoccin A stabilisation of 14-3-3 protein interaction with p53 is not well understood.
The development of assay technologies geared toward the identification of small molecule PPI stabilisers is essential for driving drug discovery. An approach that is currently under investigation seeks to harness the complexity of protein-drug-protein ternary complexes through protein-templated synthesis.
An extension of this work will be to better understand and evaluate small molecule PPI stabilisation in a cellular context. Thus, the development of disease-relevant cell-based assays is of great interest looking into the near future.
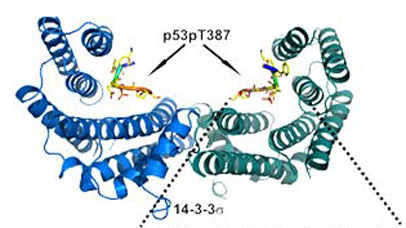
The diagram shows the backbone protein structure of 14-3-3-delta binding to part of p53, with side chains.
The diagram shows the molecular structure of fusicoccin A (C36H56O12).
The diagram shows a close up of fusicoccin A binding in the 14-3-3 p53 binding pocket (ribbon model).
Publications
Discovering Protein-Protein Interaction Stabilisers by Native Mass Spectrometry J. Bellamy-Carter M. Mohata M. Falcicchio J. Basran Y. Higuchi R.G. Doveston* A. C. Leney* Chem. Sci. 2021 12 10724-20731.
Regulation of p53 by the 14-3-3 Protein Interaction Network: New Opportunities for Drug Discovery in Cancer M. Falcicchio J. A. Ward S. Macip R. G. Doveston* - Cell Death Discovery 2020 6 126.
Adoption of a Turn Conformation Drives the Binding Affinity of p53 C-Terminal Domain Peptides to 14-3-3σ A. Kuusk J. F. Neves K. B. Rodriguez A. Gunnarsson. Y. B. Ruiz-Blanco M. Ehrmann H. Chen* I. Landrieu* E.Sanchez-Garcia* H. Boyd* C. Ottmann* R. G. Doveston* - ACS Chem. Bio. 2020 262-271.
Cooperativity in Ligand Binding between the Orthosteric and Allosteric Binding Sites of RORγt R. M. J. M. de Vries F. A. Meijer R. G. Doveston I. A. Leijten-van de Gevel L. Brunsveld Proc. Nat. Acad. Sci. 2021 118 e2021287118.
Covalent Occlusion of the RORγt Ligand Binding Pocket Allows Unambiguous Targeting of an Allosteric Site F. A. Meijer M. C. M. van den Oetelaar R. G. Doveston E. N. R. Sampers L. Brunsveld ACS Med. Chem. Lett. 2021 12 631-639.
Structure-Activity Relationship Studies of Trisubstituted Isoxazoles as Selective Allosteric Ligands for the Retinoic-Acid-Receptor-Related Orphan Receptor γt F. A. Meijer A. O. W. M. Saris R. G. Doveston G. J. M. Oerlemans R. J. M. de Vries B. A. Somsen A. Unger B. Klebl C. Ottmann P. J. Cossar L. Brunsveld J. Med. Chem. 2021 64 9238-9258.
Covalent Occlusion of the RORγt Ligand Binding Pocket Allows Unambiguous Targeting of an Allosteric Site F. A. Meijer M. C. M. van den Oetelaar R. G. Doveston E. N. R. Sampers L. Brunsveld ACS Med. Chem. Lett. 2021 12 631-639.
Ligand-based Design of Allosteric RORγt Inverse Agonists R.G. Doveston F. A. Meijer R. M. J. M. de Vries G. Vos A. Vos M. Scheepstra S. Leysen C. Ottmann L.-G. Milroy and L. Brunsveld - J. Med. Chem. 2020 241-259.
Elucidation of an Allosteric Mode-of-Action for a Thienopyrazole RORgt Inverse Agonist R. M. J. M. de Vries R. G. Doveston F. A. Meijer L. Brunsveld - ChemMedChem 2020 561-565.
Small Molecule Modulators of 14-3-3 Protein-Protein Interactions L. M. Stevers E. Sijbesma M. Botta C. MacKintosh T. Obsil I. Landrieu A. J. Wilson A.Karawajczyk J. Eickhoff J. Davis M. Hann G. O’Mahony M. Perry R. G. Doveston L. Brunsveld C. Ottmann - J. Med. Chem. 2018 3755-3778.
Supervision
I am very happy to supervise PhD students who would like to work in the field of chemical biology or medicinal chemistry. Please get in touch to discuss what projects are currently available in the group.
Teaching
I am the Chemistry MSc programme coordinator and convene the following MSc modules:
- CH7360 Advanced Research Communication and Skills
- CH7361 Advanced Practical Skills
- CH7361 MSc Research Project
Undergraduate modules:
- CH3211 Pharmaceutical Chemistry - module convenor
- CH3271 Advanced Synthetic Techniques Practical Course - module convenor
- CH4202 Advanced Synthetic Methods - lecturer
- CH3205 Metals in Synthesis (DLI ) - lecturer
I also help to deliver organic chemistry tutorials and workshops for first and second year undergraduate courses.
Press and media
Emerging concepts in drug discovery/development 14-3-3 proteins molecular glues.
Media coverage
Qualifications
Fellow of the Higher Education Academy (FHEA).