People
Dr Joanna Fox
Lecturer in Structural Biology
School/Department: Molecular and Cell Biology, Department of
Email: jf211@leicester.ac.uk
Research
Regulation and molecular architecture of apoptotic protein complexes
Regulation of the cellular life–death switch is essential in healthy cells for normal foetal development and for the clearance of damaged cells. The process of programmed cell death or apoptosis therefore has to be tightly controlled and regulated. Aberrant regulation of cell death has been implicated in numerous human diseases, including AIDS, degenerative diseases, autoimmune diseases and cancer. It is becoming increasing clear that a detailed understanding of the apoptotic signaling pathways, and the key proteins that regulate them is required if they are to be successfully modulated for therapeutic benefit.
There are two main apoptosis pathways; the extrinsic pathway is triggered by signals external to the cell binding to death receptors at the cell surface and initiating formation of the Death-Inducing Signaling Complex (DISC). The intrinsic pathway, however, is triggered by signals within the cell such as DNA damage. Intrinsic or Mitochondrial-driven apoptosis involves mitochondrial outer membrane permeabilization (MOMP) and depends on the activation of BCL-2 effector proteins, BAK and BAX. The pre-dominance of death over survival signals determines whether MOMP takes place, a process involving oligomerization and pore formation by activated BAK/BAX that releases apoptogenic factors from the mitochondria and assists the dissipation of the mitochondrial transmembrane potential, a process that is the point of no return and ultimately consigns a cell to death. BAK and BAX therefore exert their effects at a nodal commitment point in the apoptotic cascade by integrating apoptotic versus survival signals from diverse stimuli to determine cell fate.
My research focuses on regulation of the effector protein BAK. Detailed structural analysis has been undertaken to determine the conformational changes of BAK which occur to facilitate dimer and multimer formation committing the cell to undergo MOMP and apoptotic cell death. Initiation of this process is tightly controlled. I have characterised a novel phospho-regulatory mechanism involved in regulating BAK availability to become activated, and identified key proteins complexes involved in determining both phosphorylation and activation status of BAK.
I aim to establish the molecular architecture of the key protein complexes that regulate the ability of BAK to commit a cell to intrinsic apoptosis. This detailed analysis will provide mechanistic insight into the phospho-regulation of BAK and how the apoptotic threshold of cells is determined. In addition, structural studies of the protein-protein interactions may guide future drug development.
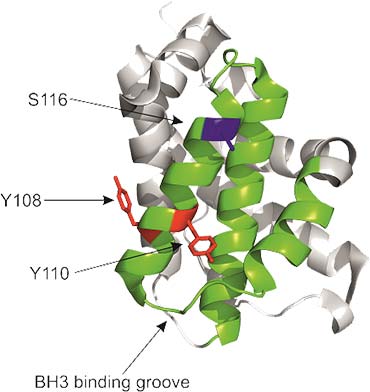
The diagram shows a ribbon presentation of BAK highlighting key residues involved in its phospho-regulation, Y108, Y110 and S116, and the BH3 binding groove.
Group members
Salma Alsubaie and John Moss (joint with Sal Macip).
Publications
Key publications
- Fox JL, Hughes MA, Meng X, Sarnowska NA, Powley IR, Jukes-Jones R, Dinsdale D, Ragan TJ, Fairall L, Schwabe JWR, Morone N, Cain K, MacFarlane M (2021) 'Cryo-EM structural analysis of FADD:Caspase-8 complexes defines the catalytic dimer architecture for co-ordinated control of cell fate' Nat Commun. 12(1):819.
- Fox JL & Storey A. (2015) 'BMX Negatively Regulates BAK Function, Thereby Increasing Apoptotic Resistance to Chemotherapeutic Drugs.' Cancer Res, vol. 75. Pp. 1345-1355.
- Azad A, et al. (2012) 'Blockade of the BAK hydrophobic groove by inhibitory phosphorylation regulates commitment to apoptosis.' PLos ONE, vol. 7. P. e49601.
- Fox JL, et al. (2010) 'Tyrosine dephosphorylation is required for Bak activation in apoptosis'. EMBO J, vol. 29. Pp 3853-3868.